Conformational Isomers
INTRODUCTION:
We have already studied structural isomerism. Isomerism of another type is stereoisomerism. Here, the arrangement of the molecules are the ones that differ. There are two types of stereoisomerism: Conformational and Configurational. Here, in this post, we will talk about conformational isomerism.
CONFORMATIONAL ISOMERISM:
In this type of isomerism, a part of a molecule is rotated by a specific angle concerning a carbon-carbon sigma bond. Conformational isomerism isn't considered to be a form of isomerism. It is still, very useful when we learn about configuration isomerism. Let us see the conformational isomerism for open-chain molecules.
CONFORMATIONAL ISOMERISM FOR OPEN-CHAIN MOLECULES:
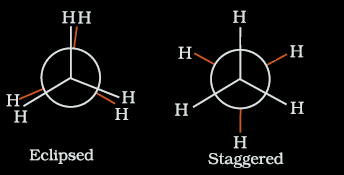
In this type of compound, Newman projections are very useful. Say we have ethane. They can be represented in the Newman projections as:
The chair form is the most stable out of all of these.



Comments
Post a Comment